บทความ
Review of literatures: Pathophysiology of dentine hypersensitivity
Introduction
Dentine hypersensitivity is an ordinary oral sensation including pain, sensitivity. The dentine hypersensitivity affects quality of life such as difficult to eating, sensitive to cold or hot water, and psychological condition (1, 2). The prevalence of dentine hypersensitivity is ranged from 3 to 57 % depending on the subjective patients’ concerning and study sample (3).
Physiology of disease: dentine hypersensitivity
Hypersensitivity of teeth is a pain sensation. This pain sensation is a sharp, shooting pain (4). Hypersensitivity indicates an abnormal pain sensation evoked by innocuous and noxious stimuli (5). The innocuous stimulus is a stimulus that is evoked by touch and air-flow. The airflow can evoke a pain sensation from prolonged or excessive stimuli. Noxious stimuli such as cold water and hypertonic solution can stimulate dentine hypersensitivity. Examples of oral diseases that cause dentine hypersensitivity are tooth surface loss and periodontal disease (6). However, dental caries, pulpitis, or defected restoration are not classified as dentine hypersensitivity (7-9).
The pathophysiology of dentine hypersensitivity is associated with the anatomy of dentine. Dentine is a particularized calcified mineral connective tissue that covered with enamel and cementum. Dentine and pulp tissue complex are responsible for nutrition and sensory function such as protect and against response to stimulus in the teeth. The dentine contains specialized cells called odontoblasts. The odontoblasts are columnar cells that originating from neural crest cells. An extension of the odontoblasts is called odontoblast process or also called Tomes's fibers. The dentinal tubule contains an odontoblast cell process and free nerve ending nociceptive receptors (10) The free nerve ending plays a role in the transduction pain mechanisms (11).
The proposed theories for dentine hypersensitivity are the odontoblastic, nerve and hydrodynamic theories (12). For the odontoblastic theory, odontoblastic processes are exposed on the dentine surface. The exposed dentine surface can be stimulated by chemical and mechanical stimuli. For the nerve theory, the presence of unmyelinated nerve fibers and neurogenic peptides at the odontoblast process receive external stimuli leading to the development of dentine hypersensitivity (13, 14). Nowadays, the hydrodynamic theory is proved by Brännström and mostly supported dentine hypersensitivity. The changes in fluid movement are caused by physical changes such as thermal stimulus, mechanical stimulus, and osmotic changes. All of the stimulations cause the movement of dentinal fluid and consequence to stimulate a physiological detector in the odontoblast. This process leads to a neural discharge response to pain signaling (15, 16).
However, the nerve theory and odontoblastic theory are associated with hydrodynamic theory. The proposed mechanisms of three theories cannot be distinguish (14). The behind mechanisms of these theories are the presence of nerves and odontoblastic process within the dentinal tubules, bathing in the dentinal fluid, and also odontoblasts are close apposition subodontoblastic plexus. Therefore, the fluid movement can stimulate the ion channels, present in the nerve ending innervation and the odontoblast in the dentinal tubule, that causes pain transduction.
Therefore, the opening in the dentinal tubules and exposure of dentine are main causes for the development of dentine hypersensitivity (2, 7, 15, 17, 18). First, the opening of tubules are caused by the loss of smear layer and enamel (19). Normally, the smear layer is a physical barrier that declines a penetration of external environment into dentinal tubules (20). However, a smear layer which is a thin structure (thickness of smear layer around 1- to 2-µm) covered of dentinal tubules (21). The loss of smear layer is caused by improper brushing, tooth surface loss and scaling and root planning debridement. The loss of the smear layer might cause an activated dentine permeability. The increased dentine permeability causes an elevated movement of dentinal fluid. An elevated movement of dentinal fluid generates an initiated action potential at the free nerve ending which develops pain hypersensitivity (22). Secondly, the sensitized dentine exposure causes a stimulated free nerve ending innervated odontoblast in dentinal tubule. The odontoblasts are developed receptor potentials and consequently the generation of action potential pass through the trigeminal pain system (23)
Moreover, the pathology of dentine hypersensitivity might depend on the size of exposed dentinal tubule. The recent review found that a sensitive tooth has a greater number of open tubules per unit area when compared to a nonsensitive teeth (17, 24). The flow of fluid movement relies on the radius of dentinal tubules which is approximately the biquadrate of a radius of tubules (25). The mean diameter’s tubules in sensitive teeth is approximately twice large when compared to nonsensitive teeth (26). Therefore, the fluid flow in tubules of sensitive teeth should be 16 times when compared to the nonsensitive teeth (27).
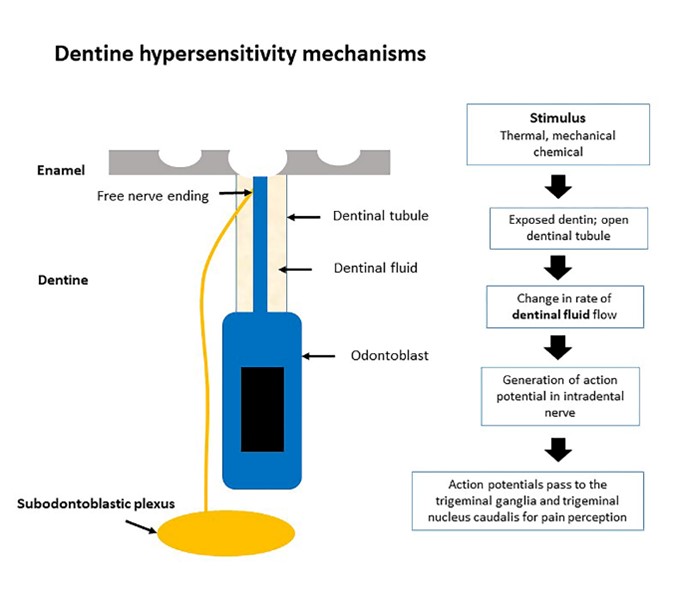
Figure 1: Focus on pathophysiology of dentine hypersensitivity
Conclusion
Sharp or sudden pain is the main symptom of dentine hypersensitivity in the teeth. This symptom is initiated by activated noxious stimuli. Pathophysiology of dentine hypersensitivity involves in exposed dentine and opening of dentinal tubules. The opening of dentinal tubules causes a response immune system that produces odontoblasts and changes the dentinal fluid. Repetitive external stimuli and the opening of dentinal tubules lead to the augmented pain action potential and the development of dentine hypersensitivity.